Dec 12, 2025
Media release
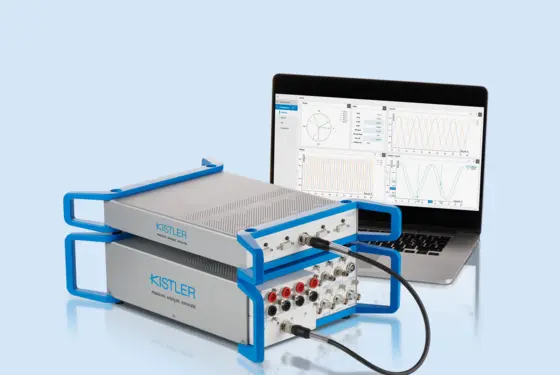
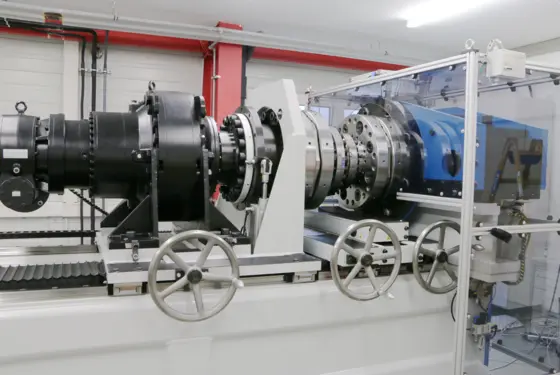
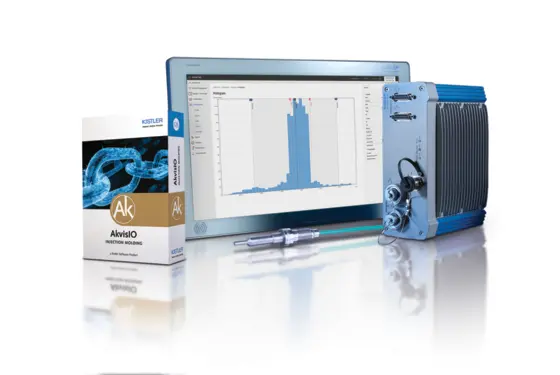
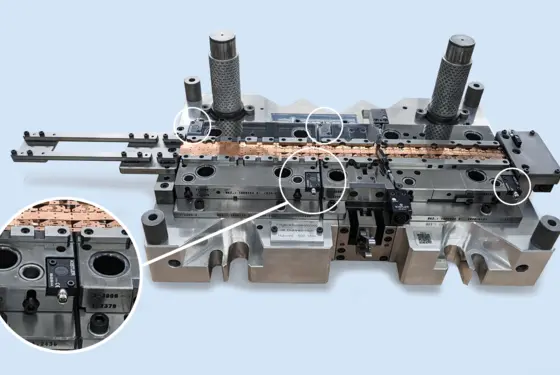
Process monitoring in joining and assembly: quick data analysis with the maXYmos Analyzer software
Optimize your production and prevent quality issues through data-driven decisions based on analyses

Keep yourself informed – learn more about the Kistler Group.