New medications are available to treat diseases such as diabetes and Parkinson's – and more people who depend on them. As a result, the demand for certain medical devices, such as auto-injectors, is rising. The medical device industry needs rapid ramp-ups of additional production capacities to keep pace with the growing demand without compromising quality. If, for example, an insulin pen does not work reliably, the consequences can be life-threatening. Manufacturers need to protect themselves as well: in case of a malfunction, they must provide proof that they have exhausted all technical possibilities to prevent production errors. State-of-the-art technology now offers significantly more thorough options than statistical process control via physical sampling, which is still widely used in the medical device industry.
Kistler presents AI-supported automated quality control in injection molding for MedTech
Winterthur, September 2025 – Quality assurance in medical technology is undergoing a radical change: away from random sampling and toward automated 100% inspection. This involves automated quality control of each individual part using AI-supported calculation of key product characteristics. The inspection is based on data, such as cavity pressure, which is taken directly from the injection molding tool. Find out more about innovative solutions from Kistler at K 2025 (Düsseldorf, October 8-15, hall 10 / booth F51).
Advanced quality assurance in injection molding for MedTech
In automated 100% inspection, every single part is checked. An AI model uses data from sensors in the mold to calculate important quality parameters such as the dimensions and weight of each individual produced part. In plastic injection molding, the well-established cavity pressure measurements and contact temperature form the basis for these calculations. Often, switching to the new method is merely a question of software and a change in the interaction between production and quality assurance. “We at Kistler are seeing more and more manufacturers from the medical industry turning to state-of-the-art production processes with automated quality control to ensure maximum safety during rapid ramp-ups. Our experts provide support in implementing this new form of quality assurance as quickly and efficiently as possible,” explains Dr. Oliver Schnerr, Head of the Business Unit Plastics at Kistler.
Successful, automated quality control with AI
Kistler will be showcasing its complete measurement chain for automated quality control at this year's K – from sensors to process monitoring and control to data documentation. Among other things, visitors will be able to see the smallest combined cavity pressure and temperature sensor (6188). The data collected by the sensor is evaluated and analyzed by Kistler's ComoNeo process monitoring system, which collects pressure and temperature data in real time throughout the entire injection molding cycle. By comparing it to reference curves, ComoNeo reliably detects deviations.
A suitable solution for automated quality control is the additional software feature ComoNeoPREDICT in combination with the STASA QC software, which performs model analyses for process validation. Based on these analyses, ComoNeoPREDICT uses artificial intelligence to calculate the quality of each individual part. The AkvisIO process data management platform documents the results and consolidates them with data from other sources. In addition to documenting processes and quality, AkvisIO allows process data to be analyzed over longer production runs and periods of time. This enables medical technology manufacturers to perfect their processes while ensuring that they comply with the highest FDA and MDR standards and deliver safe products to their customers.






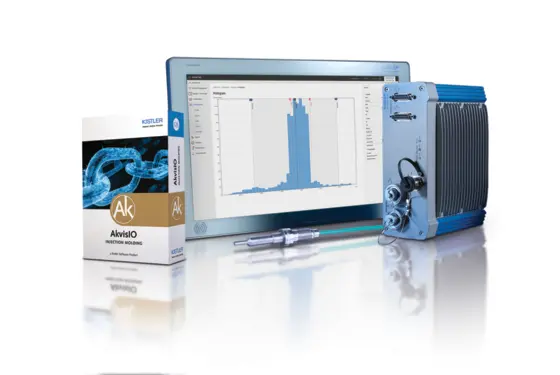
![Kistler presents AI-supported automated quality control in injection molding for MedTech [object Object]](https://kistler.cdn.celum.cloud/SAPCommerce_Document_Preview/999-345e.webp)